Recent News
New director will enhance interdisciplinary engineering learning opportunities
July 2, 2025
Final SIRI cohort visits UNM campus
June 30, 2025
Perfetti receives ANS Landis Engineering Achievement Award
June 26, 2025
Engineering a new treatment for ovarian cancer
June 24, 2025
News Archives
Hydrothermally Stable Oxide Supports Developed by UNM Researchers Will Lead to Improved Technology for Conversion of Biorenewables
October 30, 2012
 10-30-12 – The transition from diminishing petroleum-based feedstocks to sustainable biomass-derived oxygenated feedstocks in producing fuels and chemicals has gained interest worldwide. Such biorenewable conversion processes operate under aqueous-phase conditions, generally at temperatures in excess of 473 K, since biomass derivatives are highly oxygenated and soluble in water.
10-30-12 – The transition from diminishing petroleum-based feedstocks to sustainable biomass-derived oxygenated feedstocks in producing fuels and chemicals has gained interest worldwide. Such biorenewable conversion processes operate under aqueous-phase conditions, generally at temperatures in excess of 473 K, since biomass derivatives are highly oxygenated and soluble in water.
Conventional heterogeneous catalysts developed for the petrochemical industry are not suitable for biomass conversions. The current generation of catalysts is based on porous metal oxides but, while these materials are stable in gas-phase reactions, they are subject to hydrolytic attack and lose surface area and catalytic reactivity with time-on-stream.
---------------------------------------------------------------
Image:
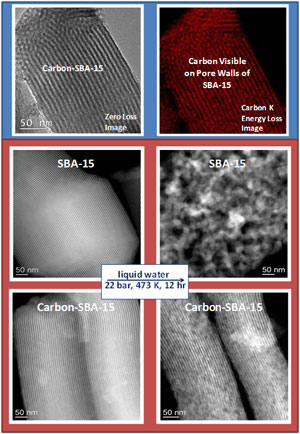
1. The image set shows a mesoporous silica SBA-15 coated with a thin film of carbon. The carbon is not visible in the bright field image, but can be seen in the Energy Filtered TEM image shown on the right.
2. The second set of four images shows how this technology improves the hydrothermal stability of mesoporous silica. After treatment for 12 hours at 22 bar pressure and 473K, the uncoated silica disintegrates and loses its pore structure. The carbon-coated silica maintains its integrity and surface area. This work will soon be published in Angewandte Chemie International Edition
---------------------------------------------------------------
On the other hand, carbon supports are hydrothermally stable at elevated temperatures and are not susceptible to degradation under aqueous conditions due to their hydrophobicity. However, carbons do not have the desired porosity or mechanical strength that is needed for catalyst supports. Therefore, a challenge for the production of biomass-derived fuels and chemicals is the development of hydrothermally stable catalysts and supports that are suitable for biorenewable processing.
 Researchers at the University of New Mexico’s (UNM) Center for Micro-Engineered Materials and Department of Chemical & Nuclear Engineering, led by Distinguished Professor Abhaya K. Datye, have developed a simple, cost-effective, and universally applicable route to produce hydrothermally stable mesoporous oxides for biorenewable conversion processes under aqueous-phase conditions at elevated temperatures.
Researchers at the University of New Mexico’s (UNM) Center for Micro-Engineered Materials and Department of Chemical & Nuclear Engineering, led by Distinguished Professor Abhaya K. Datye, have developed a simple, cost-effective, and universally applicable route to produce hydrothermally stable mesoporous oxides for biorenewable conversion processes under aqueous-phase conditions at elevated temperatures.
 This work was performed by Research Professor Hien Pham, chemical engineering undergraduate student Amanda Anderson, and two collaborators at Iowa State University (Professor Klaus-Schmidt Rohr and graduate student Robert Johnson).
This work was performed by Research Professor Hien Pham, chemical engineering undergraduate student Amanda Anderson, and two collaborators at Iowa State University (Professor Klaus-Schmidt Rohr and graduate student Robert Johnson).
 This research is a collaborative effort performed with financial support from the Center for Biorenewable Chemicals (CBiRC), a National Science Foundation Engineering Research Center (ERC). UNM is a partner in CBiRC which is based at Iowa State University. The research was also partially supported by the NSF Partnership for International Research and Education for Molecular Engineering of Catalysts for Conversion of Biomass Derived Reactants to Fuels, Chemicals and Materials.
This research is a collaborative effort performed with financial support from the Center for Biorenewable Chemicals (CBiRC), a National Science Foundation Engineering Research Center (ERC). UNM is a partner in CBiRC which is based at Iowa State University. The research was also partially supported by the NSF Partnership for International Research and Education for Molecular Engineering of Catalysts for Conversion of Biomass Derived Reactants to Fuels, Chemicals and Materials.
Professor Datye and his group prepared hydrothermally stable oxides by coating the surface of oxides with thin films of carbon, using inexpensive carbohydrates as carbon precursors. The oxides include silica and alumina, which are among the most commonly used supports in the industry but which are susceptible to degradation under hydrothermal conditions. They have demonstrated that carbon-coated mesoporous oxides have superior hydrothermal stability compared with uncoated mesoporous oxides after treatment in liquid water at 473 K for several hours, with no observed degradation or loss of surface area after hydrothermal treatment.
Coating oxides with carbon changes the surface chemistry making the surface more hydrophobic, and the oxide surface chemistry is reflected in the catalytic performance. One example is in the improved ethylene selectivity in acetylene hydrogenation for palladium (Pd) deposited on carbon-coated oxides than on uncoated oxides, with ethylene being among the high volume industrial chemicals in the United States. The major impact of using the simple carbon coating approach is that Professor Datye and his group can develop hydrothermally stable carbon-coated oxides with unique physical and chemical properties that are not possible with carbons or oxides by themselves. A hydrothermally stable oxide is necessary for achieving stable reactivity for metal catalyzed reactions, and future work will explore the applications of their carbon-coated oxides for aqueous-phase reactions.
This work has been accepted for publication and will soon appear in the journal Angewandte Chemie International Edition because of its potential high impact in the field of biomass conversion and aqueous phase catalysis. STC.UNM, the technology-transfer office at the University of New Mexico, has also filed a provisional patent application to help commercialize this technology.
