Recent News
Final SIRI cohort visits UNM campus
June 30, 2025
Perfetti receives ANS Landis Engineering Achievement Award
June 26, 2025
Engineering a new treatment for ovarian cancer
June 24, 2025
UNM engineers win 2nd place at RMWEA design competition
June 20, 2025
News Archives
Innovation also uses less platinum, expensive component of catalytic converters
December 14, 2017 - Eric Sorensen, Washington State University
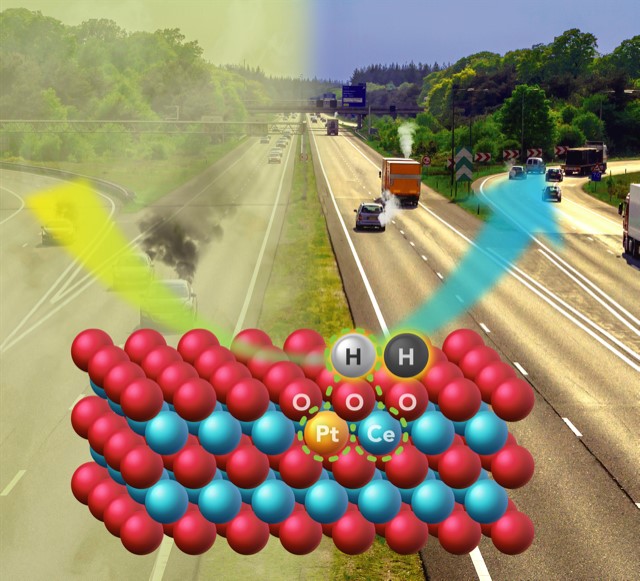 As cars become more fuel-efficient, less heat is wasted in the exhaust, which makes it harder to clean up the pollutants being emitted. Researchers at Washington State University and The University of New Mexico have created a catalyst capable of reducing pollutants at the lower temperatures expected in advanced engines.
As cars become more fuel-efficient, less heat is wasted in the exhaust, which makes it harder to clean up the pollutants being emitted. Researchers at Washington State University and The University of New Mexico have created a catalyst capable of reducing pollutants at the lower temperatures expected in advanced engines.
Their work, published this week in the journal Science, presents a new way to create a more powerful catalyst while using smaller amounts of platinum, the most expensive component of emission-control catalysts.
Catalysts have been an integral part of diesel- and gasoline-powered engines since the mid-1970s as federal regulations called for reductions of carbon monoxide, hydrocarbons and nitrogen oxides. Catalytic converters convert the pollutants to benign gases such nitrogen, carbon dioxide and water.
The researchers addressed the daunting challenge of designing a catalyst that could endure engine exhaust temperatures of up to nearly 750 degrees Celsius (about 1,500 degrees Fahrenheit) encountered under high engine loads. Yet the catalyst would still have to work when an engine is started cold and must clean up the exhaust before reaching 150 degrees Celsius, more than 100 degrees Celsius less than current systems. The lower operating temperatures during cold start are due to increasing fuel efficiency in advanced combustion engines, which leaves less energy in the tailpipe exhaust, said Abhaya Datye, a distinguished professor at UNM’s Department of Chemical and Biological Engineering and study co-author.
The recent findings grew out of a collaboration between research groups led by Yong Wang, who holds a joint appointment in Washington State University’s Gene and Linda Voiland School of Chemical Engineering and Bioengineering and the Pacific Northwest National Laboratory, and Datye’s catalysis group at UNM.
The work builds on research, published in Science last year, in which the Wang and Datye groups found a novel way to trap and stabilize individual platinum atoms on the surface of cerium oxide, a commonly used component in emissions control catalysts. The so-called single-atom catalyst uses platinum more efficiently while remaining stable at high temperatures. Platinum typically trades at prices close to or even greater than gold.
For their latest paper, the researchers steam-treated the catalyst at 750 degrees Celsius, nearly 1,400 degrees Fahrenheit. This made the already stable catalyst become very active at the low cold-start temperatures.
“We were able to meet the challenges of both the high-temperature stability and the low-temperature activity,” Wang said.
The work was funded by the U.S. Department of Energy.
